The Industry Association
Who we are
Nuclear Medicine Europe (ex AIPES) is a European Industrial Association working on promotion, awareness and defence of Nuclear Medicine and Molecular Healthcare in Europe. We are active in the field of Imaging and Therapy with Molecular and Radioactive Tracers. The main objective of our association in this field is to ensure the promotion of the economic and/or commercial interests of its Members, in particular, by all means allowing to increase the awareness to the benefits of the products and services they offer.
In addition to the main activity described here above, our association may represent the common interest of the Members in relation with the European Institutions, with other national and international authorities and in dealing with other scientific, educational or professional association groups or societies, such as EANM, EFPIA, CERN.
The Association does not interfere in the activities of its Members.
Mission Statement
“Imaging & Therapy with Molecular and Radioactive Tracers”
Our Objectives:
1. Foster Innovation in nuclear medicine and molecular imaging;
2. Cooperate for appropriate regulations in all aspects of nuclear medicine missions;
3. Support and facilitate logistics and operations needs of our members;
4. Promote the value of nuclear medicine achievements;
5. Harmonization through Europe;
6. Simplification of rules and procedures.
NMEU Position on Radiopharmaceuticals: Three Pillars to Drive our Actions:
PATIENTS SAFETY
Full compliance: adoption of all relevant requirements, both on radioactivity and pharmaceuticals Compliance covers the entire process: from R&D, to manufacturing, to distribution, to any after sale events
INNOVATION
Tradition of innovation and commitment to novel, future tracers Development of new drugs will require a more tailored regulatory approach
HEALTHCARE FOR EVERYONE
Ensuring products and technology availability for millions of procedures all over Europe.
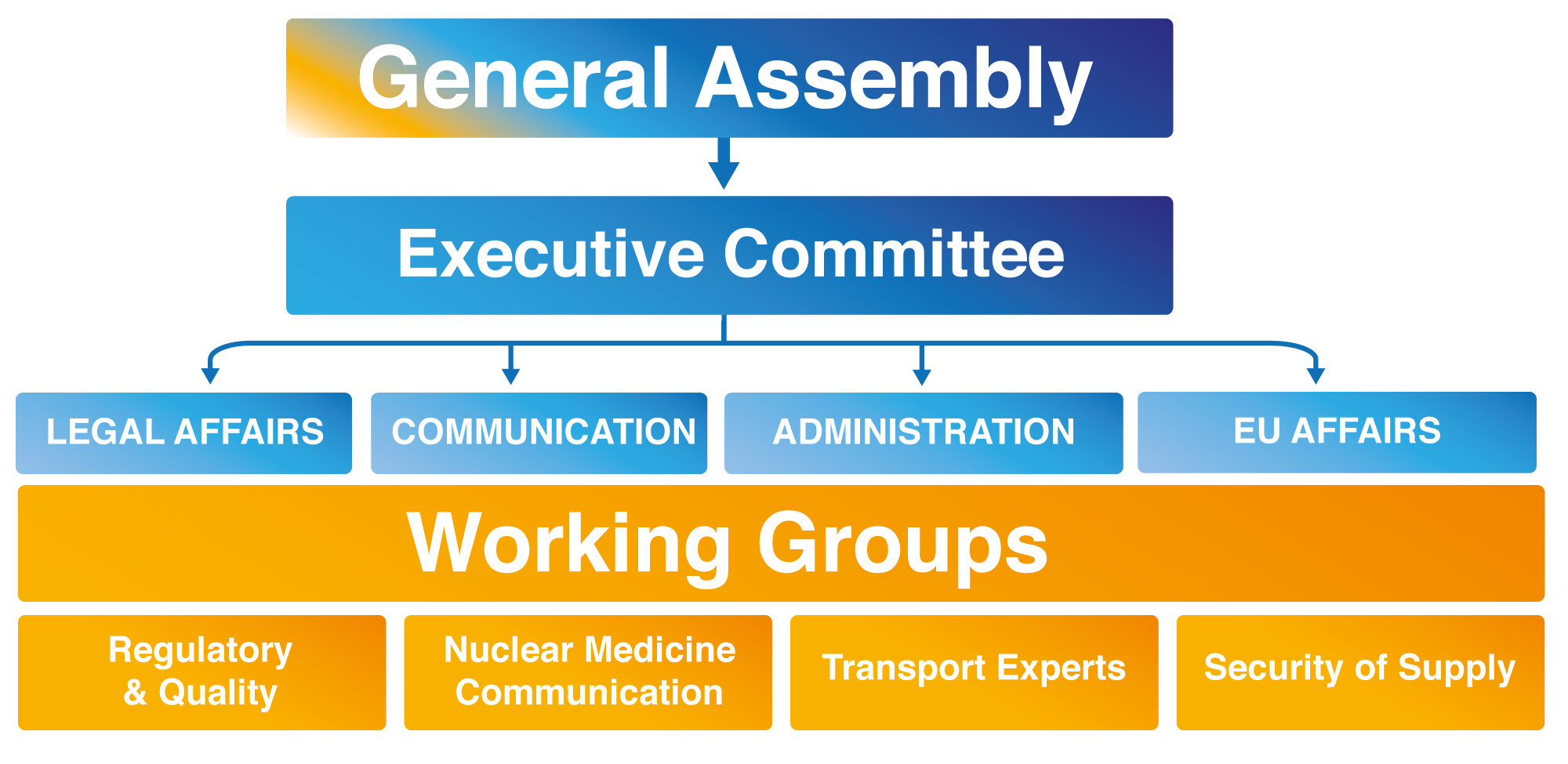
General Assembly
The General Meeting is composed of all the Members of the Grouping. Each member shall appoint an authorized representative to attend and vote on its behalf at a General Meeting. The General Assembly adopts resolutions, elects Managers of the Association and agrees to the admission of new Members. The General Meeting is composed of appointed representatives from each Member or Associate partner of the Association. Each representative has one vote and resolutions shall only be adopted by unanimous consent.
The members of the Grouping can, by a written resolution taken by unanimous consent, take all decisions which are subject to the General Meeting, out of a meeting, except for those decisions for which the adoption before a notary must be provided. Following the adoption of a resolution, the Executive Committee shall ensure that the statutory filing requirements are complied with.
The representatives of the Nuclear Medicine Europe General Assembly are :
- AAA (Advanced Accelerator Applications) — Joshua Nutting, Managing Director Radiopharma; to Deborah Langhammer until further notice; Susanne Mundo
- ARRONAX — Tony Prezeau, Directeur des Partenariats; Alternate: Ferrid Haddad; Jean-François Gestin
- ATONCO — Sylvain Fanier, President; Adrien Reymond
- BAYER AG (Radiopharmaceuticals) — Nelson Ambrogio, President Radiology; Alex Gibbs
- BLUE EARTH Diagnostics Ireland Ltd. — Matthew Morrison, Commercial PET Director; Clare Gidley
- COMECER S.p.A. — Simone Volpi, President; Martina Santandrea; Mario Malinconico
- CURIUM Netherlands BV — Timothée de Courlon, Vice President Strategy Europe; Alternate: Roy Brown, Vice President Government Affairs & Strategic Alliances; Andre Broersen
- ECKERT & ZIEGLER Radiopharma GmbH — Dirk Becker, Director Operations; Jan Schöpflin
- FRAMATOME — Ralf Gathmann, Vice President Framatome; Dominique Geslin
- GE Healthcare — Revital Melzer, Global Sourcing MI-Isotopes, PET, Generators; Alternate: Etienne Boquien, Global Business Leader Core Molecular Imaging
- IBA S.A. — Charles Kumps, Chief Innovation & Development; Alternate: Jean-Michel Geets
- IRE (Institut National des Radioéléments) — Erich Kollegger, CEO
- ITM Isotope Technologies — Roger Estefanos, Biotech Executive / Theranostics
- Johnson & Johnson Innovative Medicine — Rob Vermeulen, Director Regulatory Affairs
- LEMERPAX — Pierre-Marie Lemer, President; Christophe Selliez, Alternate; Valérie Chevreuil
- LANTHEUS EU Limited — Ira Goldman, Vice President Global Public Policy and Government Relations
- LIFE COURIERS — Claude Poliart, Group Chief Executive Officer
- LIFE MOLECULAR IMAGING — Lutz Dinkelborg, Managing Director; Nico Beukman
- LILLY FRANCE (Avid Radiopharmaceuticals) — Angel Hijos, Director European Manufacturing Operations
- MEDIRADIOPHARMA — Gyozo Janoki, Chairman and CEO; Melinda Kovacs
- ORANO CHEMISTRY ENRICHMENT — Laurent Bigot, Head of Stable Isotopes Division; Elisabeth Guillaut
- ORANOMED — Arnaud Lesegretain, President and CEO; Sophie Letournel; Elisabeth Guillaut
- PHSE snc — Paul Rice, Chief Development Officer; Hans Van de Maele
- POLATOM (National Centre for Nuclear Research) — Tomasz Dziel, Managing Director
- ROTEM GmbH — Julia Möbius, General Manager
- ROTOP PHARMAKA GmbH — Jens Junker, Managing Director
- SHINE Technologies (Shine Europe B.V.) — Harrie Buurlage, Vice President Strategic Alliances
- SIEMENS Healthineers — Deborah Langhammer, Vice President Molecular Imaging Europe; see also Advanced Accelerator Applications
- SWAN ISOTOPEN (succession of Swan Isotopen AG) — Konrade von Bremen, Chief Executive Officer
- TEMA SINERGIE — Matteo Morini, International Sales Manager
- TELIX Belgium — Raphael Ortiz, Chief Operating Officer
- TRASIS — Jean-Luc Morelle, CEO; Corentin Warnier
- URENCO Stable Isotopes — Linda Ashton, Head of Isotopes
Executive Committee

Mart-Jan Blauwhoff
President
Dutch citizen living in Germany since 1990. Married and grandfather of a beautiful and very lively young girl.
Certified Diagnostic Radiology and NM technologist (at BSc level) at Hospital of the Free University in Amsterdam.
Involved in the nuclear medicine industry, in marketing, sales and business development positions since 1984 for Mallinckrodt, Tyco Healthcare, Covidien and Curium. Worked for these companies in Netherlands, France, Belgium and Germany, currently in a European business development and marketing role for Europe. Since 2013 active member of several NMEU WGs and until June 2022 co-chair of the WG communication. In these roles contributed to many initiatives and position/white papers for Nuclear Medicine Europe.
Assigned as expert for NM at the EU BEATING CANCER PLAN COMMITTEE a European Union Special Committee on Beating Cancer (BECA) since October 2020 NM. Co-authored amendments to the EU BECA initiative on behalf of Nuclear Medicine Europe.
President of Nuclear Medicine Europe since 2022 with a reelection at the General Assembly in 2025.
In my spare time I like skiing, hiking, cooking, Cologne Karneval (Active officer of KG Treuer Husar 1925) and reading real books.

Erich Kollegger
Vice President and Secretary General
Erich Kollegger took over the General Management of the Institute for Radioelements (IRE) and its subsidiary IRE Elit early 2018. Engineer in Electro-Mechanics by education, his professional experience includes Project Management, as well as Production and Plant Management in chemical and pharmaceutical plants, in Belgium and France. Before joining IRE, Erich was Plant Manager of a manufacturing site for Baxter Healthcare in Lessines, Belgium. Erich is also Chairman of the Board of Directors of Transrad, a company specialized in transport of radioactive material, and represents IRE as director of Sterigenics and Oncidium.
He is appointed lecturer at the Universities of Liège, Brussels and Louvain-la-Neuve in Belgium, being in charge of a course on Business management.

Konrade von Bremen
Vice President and Treasurer
Coming soon

Matt Morrison
Vice President
Matt Morrison is currently the VP International Markets at Blue Earth Dx Ltd, a Bracco Company.
He has been in the nuclear medicine industry since joining the Amersham radiopharmaceutical business as an R&D scientist in 1998.
Matt spent many years working on the development of novel SPECT and PET tracers, prior to moving into a commercial product management role at GE Healthcare (focused on nuclear cardiology), before joining Blue Earth Dx Ltd in July 2021 to lead their International commercial efforts.
Matt has a BSc in Toxicology & Pharmacology and a PhD.
Matt has been part of the Nuclear Medicine Europe team for several years as a company representative.
He lives in Amersham, UK with his wife and three children.

Etienne Boquien
Vice President
Etienne Boquien has been with GE HealthCare since 2005 and has played a role in the nuclear medicine industry since 2008. He currently leads the Global Core Molecular Imaging business, driving innovation and strategic growth in this critical area of healthcare.
Before joining GE, Etienne held finance leadership roles at Eagle Pitcher in Canada and Alcatel in Italy, gaining valuable international experience in corporate finance and operations.
With a strong background in product management, corporate finance, and strategic project leadership, Etienne brings a unique blend of business acumen and technical insight to his role. Since 2022, he has also been an active contributor to the Nuclear Medicine Europe Communication Working Group, reflecting his commitment to collaboration and cross-functional engagement.
Fluent in French, English, and Italian, Etienne offers a multicultural perspective and a collaborative leadership style that aligns with Nuclear Medicine Europe’s pan-European vision.
He lives just west of Paris with his wife and two daughters.

Guy Turquet de Beauregard
Honorary President
Guy Turquet de Beauregard, who has been a member of the Executive Committee since 2008 and President of AIPES from 2013 to 2016, devoted his professional life to nuclear medicine and has always been a strong supporter of our industry became AIPES Honorary President. AIPES continues to benefit from his valuable advice and contacts in the scientific and political world.
Executive Office - Brussels
The administration office, based in Brussels, is in charge of coordination between stakeholders, and acts as a liaison office and an information platform between the Group and the other European entities.
David Crunelle is a multi-award winning designer specializing in visual communication. He taught communication in higher education for more than 10 years, was a photographer for Getty, KOL and influencer for several tech brands, and is recognized in the contemporary artistic world. In addition to being a consultant for more than 35 European associations, he has worked closely with AIPES/NMEU since 2008.
Jocelyne Baldasso
Executive Administrator
Based in the NMEU Executive Office – Brussels since 2006, Jocelyne Baldasso is in charge of the coordination and communication for the European activities and the management of the financial operations of the Association.
Jocelyne has an extensive experience at corporate, governmental, and executive levels within international companies and European Associations. She holds a degree in psychology of sciences of human behavior (awarded in 1998).
Code of Ethics
The sensitive nature of NMEU activities for the public, (i.e. human healthcare issues and radioactive hazards fear), implies at any time the risk of being the target of public inquiry and questioning. Moreover, NMEU wants to promote fair competition between its members. NMEU members should therefore at least fully comply with the EU and International legislation and principles related to:
- Good Manufacturing Practice and Good Clinical Practice
- Radioactive Transport Regulation
- Radioprotection regulations for products or employees
- Confidentiality of information
- Patent protection
- Fair Trade
- Each company should respect EU (and related EMEA and/or MDD guidelines) and local Good Manufacturing Practice (GMP) rules in the production of radiopharmaceuticals and medical equipment.
- Each company should respect EU (and related EMEA guidelines) and local GMP rules for the production of Investigational Medical Products (IMP) for human use.
- IMP for human use should only be provided to customers within the framework of a clinical study or for compassionate use and, in any case, under the express authorization from the competent authority responsible for IMP for human use
- No company shall sell, distribute or transport products from producers which do not respect EU and local GMP rules for the production of radiopharmaceuticals and medical equipment.
- No company shall sell, distribute or transport Investigational Medical Products (IMP) for human use not produced in laboratories, which are not authorized for IMP.
- Each company should strictly respect ADR regulations (European Agreement concerning the International Carriage of Dangerous Goods by Road) when transporting radiopharmaceuticals and IMP.
- Regarding their employees and products, each company should comply with ICRP (International Commission on Radiological Protection) and local radioprotection rules in the production, distribution and transportation of radiopharmaceuticals.
- Each company should report to NMEU any event that could trigger a crisis communication plan from the NMEU Executive Committee, in particular in view of avoiding potential EU-wide negative impact for the industry and/or customers.
- Each company should respect confidentiality of information and documents shared within NMEU, during and from NMEU meetings. Such confidential information cannot, in any case, be used against any NMEU member.
- Each company should comply with patents regulation.
History
Emanating from the first clinical use of nuclear medicine tracers and therapeutics in the 1950s, a clinical discipline and supporting industry began to emerge during the 1970s in the United States, Europe and Japan. By 1985 this activity was considered appropriate for regulation by the European Union, and a Directive bringing radiopharmaceuticals in line with all other drug substances was introduced.
In 1987, all interested radiopharmaceutical companies agreed to form the Association of Radiopharmaceutical Producers Europe (ARPE) which was formalised shortly afterwards as a European economic interest group (EEIG).
ARPE’s initial focus on pharmaceutical regulations was very successful and led to an agreement with the EEC for rational registration process. In addition to these activities ARPE also became a focal point for dealing with threats to the continuing success of radiopharmaceuticals in Europe. For example, a threat to disrupt supply of a key raw material in the production of radiopharmaceutical preparations was addressed by focussed lobbying with governments, regulators and industrial partners.
By the mid 1990s the professional organisation representing nuclear medicine physicians in Europe (EANM) provided an opportunity for ARPE to become directly involved in a strategic collaboration to develop the speciality. In order to appropriately respond to this challenge, ARPE broadened its statutes to encompass hardware and equipment companies and renamed itself the Association of Radiopharmaceutical Producers and Equipment Suppliers (ARPES). ARPES was closely involved with several EANM projects including the funding of a cost benefit study for nuclear cardiology and a contribution to the establishment of a strategic vision for nuclear medicine in Europe. By the end of the 1990s most members had broadened their scope and were now engaged in multi-modality in-vivo diagnostic activities.
To reflect this reality a new name AIPES (Association of Imaging Producers and Imaging Suppliers), with the mission to foster industry collaboration across all fields of in-vivo diagnosis, was adopted in 2001.
During its relatively brief history our industry association has experienced massive technological, commercial and regulatory change. Despite this change, our members remain committed to providing the highest standards of product and service excellence to physicians worldwide and ultimately the patients that they serve.
In May 2019, AIPES changed its name and visual identity to become Nuclear Medicine Europe.