Nuclear Medicine Europe
Activity Report 2024
Another stage on the path of our growth
Word of the President
Dear Colleagues,
As we just completed the year 2024, the Chinese wisdom that “nearing completion” indicates there is still much to be done is particularly apt.
Taking the pen to write the greetings for the annual news is always an exciting moment. Being an active part in this vibrant field of today’s medicine is thrilling and challenging at the same time. We look back and realize the number of events, activities which were accomplished and the important decisions taken.
Our association is increasingly at the forefront of the ongoing many important changes in European healthcare.
We have a unique position, grouping all industrial players involved in nuclear medicine, from the reactors, stable isotope producers, equipment providers and radiopharmaceutical industries of all sizes. This specificity is a unique strength in the field.
We are united by the fact that we are all dealing with radioactivity for patient care. The great variety of our members is our important asset, it is also a challenge to unite so many different industrial worlds.
This year once more showed us that our presence and position are important in many different actions at the EU level. We are regularly invited to participate as representatives of the nuclear medicine industry sector in European projects and conferences.
Despite the incredible growth in the field of Nuclear medicine, our association has to face several internal difficulties.
With the rising cost of living in Europe, and with our finances remaining unchanged, it is thanks to our colleagues, teams, and working groups that we have managed to make a significant impact on the European and international stage.
I will not wait until the end to acknowledge the ingenuity and dedication of those within NMEU who have given their time, motivation, and initiative to realize the projects we have participated in, often under challenging conditions. Their achievements are detailed further in our newsletter.
We could have accomplished more, but we lacked the necessary resources and had to make restrictive choices, such as relinquishing our annual symposium, despite its gaining success.
As the year comes to a close, we now focus on the actions to undertake starting in 2025. Certainly, our financial situation remains challenging, with a minimum 4% increase in our fixed costs from the beginning of January.
We are determined to maintain the quality of our exchanges, and objectives, and to impose our vision of nuclear medicine on the profession and the general public. We have to start the year by considering all possible economies This is a challenging exercise, but we will strive to achieve the impossible!
Our core team remains at the helm:
• Guy Turquet de Beauregard, our indispensable Honorary President, who with his well-known sense of diplomacy, ensures a seamless link between the past, present, and the future of our discipline.
• David Crunelle, whose professionalism in communications has elevated our image to the level of major international associations, and whose initiatives are always warmly welcomed.
• Jocelyne Baldasso, always available regardless of the hour or time zone. Despite the growing number of our members, she remains attentive to each of your requests and ensures our connections, while taking into account the priority of your obligations within your respective companies.
Our small administrative structure could not function without the support of our lawyer, Isabelle Van Schendel, our precious expert accountant Michelle Verdeyen, and our auditor Benjamin Gorlier.
The management of our association is a team effort, and the time contributed by my colleagues Konrade von Bremen, Erich Kollegger, and Lutz Helmke is particularly appreciated, as is their sense of duty in collective conscience for the benefit of Nuclear Medicine Europe.
Thank you all.
Today, we must also acknowledge the departure of one colleague from the Executive Committee. Lutz Helmke is leaving us to enjoy a well-deserved retirement. His wise advice and guidance will be deeply missed. We wish him the best in his new life, which we have no doubt, will be filled with a lot of interests.
Beyond the customary practice of ending my speech with New Year’s wishes, I would like to emphasize the significance they carry. It is a real honour for us, the Executive Committee, to lead NMEU in the best interests of all of you. We wish that 2025 will be a year of success, productive exchanges, and serenity in a world that sorely needs it.
Wishing you a happy, healthy and successful 2025 to you all.
Addressing Workforce Challenges
Exploring Student Motivation and Barriers
Publication / Communication
Throughout the year, Working Groups, Members, external consultants, and other specialists in their fields contribute in-depth analyses on specific topics. This year, one of the key focuses—prompted by requests from industry members—was the workforce challenges looming in the near future, particularly the lack of interest or awareness among academics regarding nuclear medicine.
To explore this issue, we engaged with Professor Serge Goldman to discuss the factors that inspire students to pursue careers in nuclear medicine, as well as the barriers that deter them from entering the field.
Additional publications on this and other topics can be found in their dedicated section.
Why do medical students decide not to get trained in the most appealing speciality, nuclear medicine?
Why do medical students opt out of training in nuclear medicine, despite its appeal?
Science, Significance, and Future Perspectives Of Nuclear Medicine
An extended dialogue with a nuclear medicine specialist and university professor.
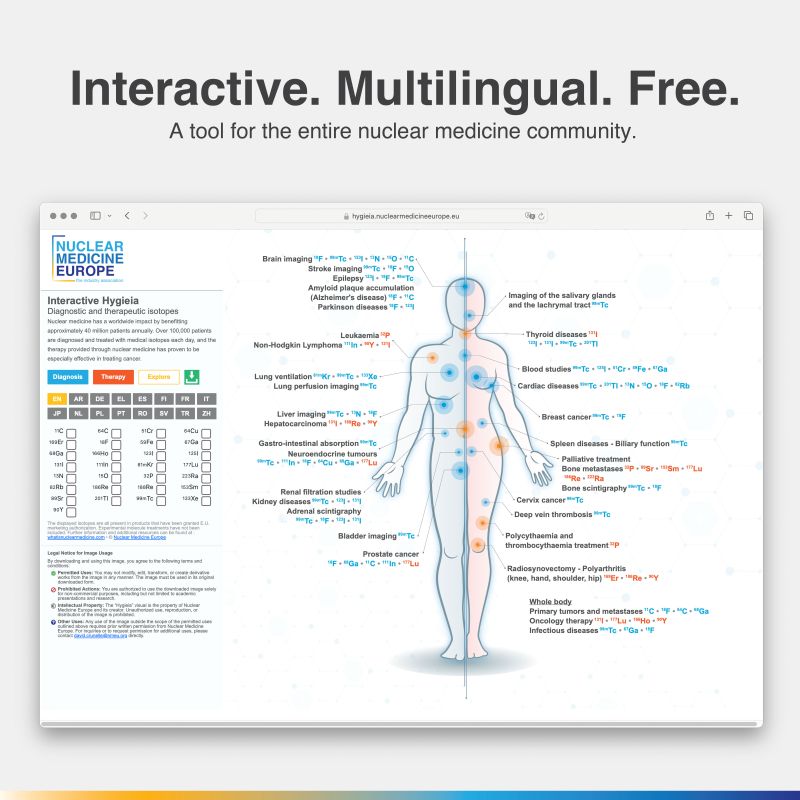
Interactive Hygieia
Global Superstar
Web app / Communication
Hygieia, Nuclear Medicine Europe’s highly popular and successful data visualization tool for radioisotopes used in therapy and diagnosis, has taken a leap forward with the launch of an interactive version. This innovative platform allows users—including physicians, academics, and the general public—to customize the poster in one of 16 languages with just a few clicks.
While we anticipated a warm reception, the response exceeded all expectations: over 10,000 posters were generated within the first 48 hours of the web app’s launch. The tool was officially introduced at the EANM Congress, where several participants shared that they were already using it in their presentations and keynotes during the event – just a few days after the launch!
The Communications Working Group welcomed the overwhelmingly positive feedback and immediately began brainstorming ways to enhance the tool further. We eagerly look forward to the next evolution of this exciting app!
https://hygieia.nuclearmedicineeurope.eu/
The association's active presence at key events
Amplifying our voice
This year again, Nuclear Medicine Europe has been actively participating in key events to be the voice of the industry, to advance cancer care and secure radiopharmaceutical supply chains across Europe. The following sections highlight the association’s involvement in significant initiatives, including the European Commission’s conference on cancer, workshops on radiopharmaceutical access, and annual gatherings to review progress and foster collaboration within the nuclear medicine community.
Europe United Against Cancer
Advocating Equity, Access, and Early Diagnosis
Congress / European Commission
01.2024

“Equity in health is non-negotiable”
– Commissioner Stella Kyriakides
“The final destination is a Europe without cancer”
– European Parliament President Roberta Metsola
Nuclear Medicine Europe was invited by the European Commission to attend the conference “Europe’s Beating Cancer Plan: Joining Forces”. Marked by impactful messages and patient testimonials, the event underscored critical themes such as “prevention” and “accessibility.” These pivotal aspects align with Nuclear Medicine Europe’s persistent advocacy for enhanced patient access and early diagnosis. A crucial step forward involves harmonizing the legislative framework across Europe to ensure comprehensive improvement.
Ensuring Radiopharmaceutical Access
Supply Chains and EU Autonomy at High-Level Brussels Workshop
Workshop / European Affairs
02.2024
In the presence of the Belgian Minister of Energy, Tinne Van der Straeten, Nuclear Medicine Europe was invited to participate in the recent workshop “Securing Access to Radiopharmaceuticals for all European Patients”, held in Brussels. This event, held under the Belgian Presidency of the Council of the European Union 2024, brought together the key players in the field. As always, our presence served as a voice for the association’s members, advocating for a crucial issue: ensuring easier access to radiopharmaceuticals for all European patients.


The session 4, “Securing the radiopharmaceutical supply chain and fostering the EU autonomy” saw the intervention of our friends Regulatory Affairs and Quality Working Group chair Cristiana Gameiro from IBA, Renata Mikolajczak from Radioisotope Centre POLATOM, Ronald Schram from NRG PALLAS, Security of Supply Working Group chair Bernard Ponsard from SCK CEN and Therapy Working Group chair Jean BONNET from IRE – Institute for radioelements.
Annual General Meeting
Reviewing Progress, Building Connections
Events / Networking
05.2024
The Nuclear Medicine Europe Annual General Assembly is traditionally held in Brussels, beginning with a dinner in the elegant and historic setting of the Solvay Library. The meeting itself took place at the Manos Hotel and Conference Center, where attendees reviewed the financial reports and presentations. Chairs of the Working Groups provided detailed updates on their activities and progress over the past year, offering details insights into the organization’s achievements and future projects.
We were again honoured to host representatives from the European Association of Nuclear Medicine (EANM) and the European Observatory, whose presence added significant value to the discussions. As always, the General Assembly was more than just a formal event; it served as a vital platform for networking. Throughout the 2-day event, members participated in side meetings, fostering collaboration and strengthening ties within the nuclear medicine community.

Euratom Supply Agency tackling the shortages of medical radioisotopes - the Observatory on the Supply of Medical Radioisotopes
By Euratom Supply Agency

The security of supply of medical radioisotopes came to public attention at the time of severe supply shortages in 2008-2009, which triggered the 2010 Council Conclusions “Towards the Secure Supply of Radioisotopes for Medical Use in the European Union“. To support the security and sustainability of supply in the EU, the Commission – in cooperation with the nuclear medicine industry organisation for Europe AIPES (later renamed Nuclear Medicine Europe) created the European Observatory on the Supply of Medical Radioisotopes (Observatory) in 2012.
Since 2013, the Observatory is run by the Euratom Supply Agency (ESA); its meetings are co-chaired by Nuclear Medicine Europe.
The Observatory focuses on the supply of Technetium-99m (Tc-99m), the most widely used (diagnostic) isotope. Europe is the second largest consumer of Tc-99m, accounting for more than 20% of the global market. The production of Tc-99m is a complex process which includes irradiation of uranium targets in nuclear research reactors to produce Molybdenum-99 (Mo-99), extraction of Mo-99 from targets in specialised processing facilities, production of Tc-99m generators and shipment to hospitals. Tc-99 is mostly produced in research reactors, mostly in the European Union.
Since 2012, the Observatory monitors the EU supply chain of Mo-99/Tc-99m. It also looks at certain aspects of the EU supply of other widely used medical radioisotopes. The Observatory brings together various supply chain stakeholders (industry, research organizations) and EU Member States, nuclear medicine organisations, members of Nuclear Medicine Europe, relevant international organisations as well as Commission services and EU Agencies.
The Nuclear Medicine Europe’s Security of Supply Working Group ensures effective coordination of reactor maintenance schedules to avoid and mitigate disruptions in the supply of Mo-99/Tc-99m. Within this working group, the Emergency Response Team (ERT) is composed of representatives of research reactors, Mo-99 processors and Mo-99/Tc-99m generator manufacturers, and monitors emerging production and supply issues. This continuous monitoring enables identifying potential shortages of Mo-99 and draws up mitigation action plans involving all stakeholders. The Observatory’s Joint Communication Team (JCT) disseminates in a timely manner the information received from the ERT to various stakeholder groups, including EU Institutions, OECD/NEA and IAEA. The ERT/JCT work has been instrumental in ensuring an uninterrupted supply of Mo-99/Tc-99m, particularly in relation to the impacts of Brexit the Covid-19 pandemic.
In March 2021, the Observatory’s mission statement was revisited and new terms of reference were drafted. The following objectives were set for the Observatory:
• to support secure and sustainable medical radioisotope supply across the EU taking into account the worldwide need and supply;
• to ensure that the medical radioisotope supply issue is given high political visibility in international and national institutions, organizations and bodies;
• to identify any event or trend likely to impact the medical radioisotope supply, including logistics, and call relevant parties to take appropriate countermeasures;
• to promptly disseminate through agreed communication channels the enquired information regarding any possible supply disruptions or other supply-related issues;
• to establish periodic reviews of the medical radioisotope supply chain and capacities with all stakeholders across the EU, taking into account the worldwide need and supply, and to forecast future needs;
• to build a foresight overview of the supply and demand of medical radioisotopes at EU level;
• to acquire the latest information on the development and implementation of new and alternative methods and technologies of medical radioisotope production.
The most recent plenary meeting of the Observatory was held on 25 September in Geneva, hosted by CERN.
The meeting provided an opportunity to hear about CERN’s role in radioisotope mass separation, as well as about the PRISMAP EC project, managed by CERN, which provides a wide range of radionuclides for medical research. The project built a strong network of world-leading European facilities, including nuclear reactors, medium- and high-energy accelerators and radiochemical laboratories.
The Observatory reviewed the global research reactor scheduling for the remainder of 2024 and 2025.
DG ENER provided an update on the latest developments related to the creation of a European Radioisotope Valley initiative in the context of the SAMIRA action plan. The European Medicines Agency presented its ongoing initiatives to secure the supply of medicines in the EU and to prevent shortages of medicinal products. DGs RTD, JRC and GROW, as well as REA, also presented their activities related to medical radioisotopes and SAMIRA.


The meeting also heard presentations from three French facilities: the Institut Laue–Langevin (ILL) research reactor, the Jules Horowitz Reactor (JHR) which is currently under construction and the Orano Stable Isotopes facility. Alongside the new Dutch research reactor Pallas, these facilities will bring more radioisotope production capacity to the market. Nuclear Medicine Europe, the European Association of Nuclear Medicine (EANM) and the OECD Nuclear Energy Agency (NEA) also presented their activities, including the upcoming Workshop on Medical Radioisotopes Supply planned on 24-25 October in Paris.
Following the meeting, a technical visit to the two CERN facilities ISOLDE and MEDICIS was held.
The next meeting will take place in the 2nd half of 2025 in France and will be hosted by the CEA Cadarache, where the Jules Horowitz Reactor is currently under construction.
Visit of the CERN facilities
In the earth of the research
Technical visit
09.2024

The participants of the European Observatory on the Supply of Medical Radioisotopes meeting had an exceptional opportunity to participate in a technical visit to the CERN facilities, guided by leading researchers through CERN’s state-of-the-art installations, providing an insider’s view of projects that are unique to this premier scientific research centre.
CERN, the European Organization for Nuclear Research, has been at the forefront of particle physics since its establishment in 1954. Home to the Large Hadron Collider (LHC), the world’s largest and most powerful particle accelerator, CERN has made monumental contributions to science, including the discovery of the Higgs boson in 2012.
Second International Workshop on Medical Radioisotopes Supply
Advancing Radioisotope Supply Chains for Revolutionary Cancer Therapies
10.2024
The second International Workshop on Medical Radioisotopes, co-hosted by the NEA, US Department of Energy (DOE), and the European Commission’s Joint Research Centre (JRC), showcased a strong presence from Nuclear Medicine Europe. Held with participation from policymakers, industry leaders, and researchers, the workshop explored critical challenges in securing sustainable access to innovative isotopes like Lutetium-177 (177Lu) and Actinium-225 (225Ac).

Presentations delved into strategies for supply chain resilience, infrastructure needs, and the role of advanced isotopes in the fight against cancer. Building on NEA’s work to stabilize radioisotope supplies, the event highlighted the collective determination to harness the full potential of nuclear medicine for cancer diagnostics and therapeutics.
You can access all the presentations through the following link.


The following document is an in-depth synthesis of the Second International OECD Workshop on Medical Radioisotopes Supply, authored by Honorary President Guy Turquet de Beauregard for Nuclear Medicine Europe members. This synthesis resumes the development of secure supply chains for conventional and innovative medical radioisotopes, particularly Lu-177 and Ac-225, emphasizing Radioligand Therapy (RLT) and the potential market for these isotopes. The document also outlines the key discussions, findings, and recommendations from the whole event. All related presentations are accessible via the OECD-NEA website.
You can access all the synthesis through the following link.
European Affairs
By Konrade von Bremen, Vice-President and EU Affairs Representative for Nuclear Medicine Europe
2024 was a very dynamic election year for Europe. In June the 26 EU member states voted for a new Parliament and European Commission President. The long-term EU projects continue their strategic path, in which Nuclear Medicine Europe is actively involved.
Nuclear Medicine Europe participates in the frame of the SAMIRA action plan for the 2023 started ERVI project (European Radioisotope Valley Initiative) as a European Commission-nominated member of the Steering group.
During the past year, three steering group meetings, in January, June and November made substantial progress in the identification, the selection and the detailing of the future ERVI projects. Nuclear Medicine Europe represents the entire industry sector in this future-shaping initiative.
It is recognized that the role of the nuclear medicine industry sector is key in the identification and in particular in the technical and economic feasibility of the big infrastructure projects to be implemented in Europe, for the sake of the security of supply of patient care. 2025 will bring the first phase of the project to its next step before implementation.
The reform of the EU pharmaceutical legislation came to a first decision in April 2024, when the departing European Parliament voted in favour of the reform of the European Pharma Law with only a minimal recognition of the growing field of Nuclear Medicine and radiopharmaceuticals. To our regret, the prepared amendments together with EANM were not accepted in this decision. The Council of Europe still needs to adopt it and will continue the debate also in 2025. There is still hope to give radiopharmaceuticals adequate recognition in the reform of the pharma law.
The experience of a worldwide pandemic has sharpened the need for the security of the European supply of critical medicines. The EU Commission and EMA launched in April 2024 the Critical Medicine Alliance. Nuclear Medicine Europe applied for membership and is actively following the development. Until today radiopharmaceuticals are only marginally present in the list of critical medicines. The focus is on the worldwide supply chain of blockbuster drugs so that Nuclear Medicine Europe could stress the fact that security of supply is one of our priorities and expertise in the field.
The European Commission together with EMA are implementing the new Health Technology Assessment Regulation, coming into force on the 12th January 2025 for all new oncology medicines and advanced therapy medicinal products. Health technology assessments are procedures for assessing the added value, effectiveness and costs as well as the broader impact of the health of a pharmaceutical. The compulsory technology assessment of all new oncological radiopharmaceuticals will be an additional challenge for the industry sector. To be able to provide information and support for its members, Nuclear Medicine Europe applied to be part of the HTA stakeholder network of the EC and EMA.
During the past year, Nuclear Medicine Europe has experienced a significant broadening of its relations with EMA. If previously Nuclear Medicine Europe had the opportunity to meet with the Industry stakeholder representative once a year and exchange on topics of mutual interest, during a strategic meeting in June 2024, Nuclear Medicine Europe was invited by EMA to take part in a number of stakeholder groups with different regulatory focus, clinical CMC, GMP and others. This gives Nuclear Medicine Europe and its members an in-depth view of EMA activities and allows the Working Group members to actively participate with other industry representatives, shaping the future of the EU pharma world. Nuclear medicine and radiopharmaceuticals are now in the focus of EMA. The Working Group RA&Q bring details to this topic below.
Collaboration and Innovation at the SNMMI
Strengthening Partnerships and Connections in Nuclear Medicine
Congresses / Events / Networking
06.2024

During the SNMMI Annual Meeting in Toronto in June, Nuclear Medicine Europe participated in the CORAR (Council on Radionuclides and Radiopharmaceuticals) Informal Meeting of Trade Associations and held a highly productive Security of Supply Working Group meeting, which included the newly joined University of Missouri-Columbia Research Reactor. This was followed by an informal gathering where Working Group members and friends enjoyed some time together.
The congress also provided an opportunity to see the latest products and developments from Nuclear Medicine Europe Members. We explored the booths of (amongst others) Comecer, Tema Sinergie, ITM Isotope Technologies Munich SE, Monrol Nuclear Products Co., RLG – RADIOPHARMA LOGISTICS GROUP, Siemens Healthineers, Curium Pharma, Advanced Accelerator Applications, IBA RadioPharma Solutions, Jubilant Radiopharma, Cardinal Health, Lantheus, but also our friends of Serac Life Sciences, the Oncidium foundation and many more.
Nuclear Medicine Europe leveraged its presence at these congresses to facilitate networking among members, discussing upcoming projects and opportunities for each working groups.
While we all spend (too) many hours in video calls, it was refreshing to meet face-to-face.
EANM Congress in Hamburg
Presence at the European congress
Congresses / Events / Networking
10.2024


The European Association of Nuclear Medicine (EANM) Congress has come to a close, and what an eventful and rewarding experience it has been this year again. It was a pleasure to connect or reconnect with so many people and Members of Nuclear Medicine Europe, even amidst the very busy schedule.
We are extremely happy with the overwhelmingly positive feedback we received regarding the Interactive Hygieia. It has resonated well with the diverse audience within the nuclear medicine community and was even featured in several panel presentations during the congress. Thank you!
If you have not tried it yet yourself, visit hygieia.site and let us know what you think of it.
A special thank you to everyone who joined us for our Get Together Meeting following the Security of Supply Working Group meeting (more details about it in our previous post). We look forward to continuing these hashtag#networking events at future working group meetings, upcoming congresses, and of course, at next year’s EANM Congress in beautiful Barcelona.


Trailblazing Trends for Tomorrow’s Nuclear Medicine
EANM Plenary Session
The EU Strategy to Guarantee the Future of Nuclear Medicine
Nuclear Medicine Europe’s EU Affairs and Vice-President Konrade von Bremen was invited by EANM organization to discuss the EU’s comprehensive strategy to secure the future of nuclear medicine, emphasizing the critical role of radiological and nuclear technology in healthcare.
Her presentation highlighted the need for reform in EU pharmaceutical legislation to better support the nuclear medicine sector. It stresses the importance of stakeholder involvement in decision-making processes and calls for recognition of nuclear medicine’s unique characteristics within the broader healthcare system. The presentation concludes with a call to action for stakeholders to engage actively in shaping policies that will enhance the sustainability and independence of nuclear medicine in Europe.
EANM-NMEU Leadership Policy Meeting
The EANM-NMEU Leadership Policy Meeting, held on October 21, 2024, in Hamburg, marked an important step in strengthening collaboration to advance nuclear medicine across Europe. Key discussions revolved around the secure and sustainable supply of medical radioisotopes, including updates on ongoing coordination efforts and communications with South African ministers regarding additional cycles for the HFR reactor. Participants also addressed the critical need to enhance access to care in light of the upcoming implementation of the HTA Regulation for oncology drugs. Recognizing the limited presence of nuclear medicine in European HTA frameworks, the meeting underscored the importance of increasing sector representation within the European Commission’s Stakeholder Network. Suggestions included developing partnerships with universities to explore health economics, expanding training opportunities for young professionals, and leveraging successful data management models such as the Belgium approach.
In addition to technical and regulatory priorities, the meeting highlighted opportunities for deeper policy collaboration. Discussions included engagement with the European Medicines Agency (EMA) on its revised conflict of interest policy and participation in the broader dialogue around the Pharmaceuticals Legislation Review. Plans were also laid for the upcoming Pharma Package meeting in Brussels, where EANM will contribute as an observer.
With a shared commitment to advancing strategic initiatives and fostering innovation, the meeting concluded with clear next steps and a renewed focus on shaping the future of nuclear medicine in Europe.
Expanding Linkedin presence
From Connection to Conversation
Communication
In 2024 again, our social media strategy has demonstrated its effectiveness, achieving consistent organic growth without any paid promotions or sponsored posts. This platform has solidified its role as a primary communication channel for our association, reaching a broad audience within the nuclear medicine community. It has also proven invaluable for disseminating sensitive information during periods of disruption (as detailed in the Security of Supply section) and serves as a trusted resource for national associations. Our goal is to continue enhancing this channel to keep it engaging and informative for our members and the wider nuclear medicine community.
Linkedin followers


Working Groups
The heart of the association
Association / Working Groups
The Nuclear Medicine Europe Working Groups play a vital role in driving progress within our organization. Committed and proactive, these teams bring expertise and dedication to advancing key initiatives that support our shared goals. Their efforts ensure the continuous improvement and relevance of nuclear medicine across various disciplines.
Below is a – very – brief summary of each working group’s activities, a snapshot that hardly captures the depth and dedication each member has poured into their individual projects. This condensed overview cannot fully reflect the significant effort and impact of their work, which deserves far more recognition than these few words can convey.
Nuclear Medicine Europe, along with the Executive Committee and countless contributors from the nuclear medicine community, extends heartfelt gratitude to each member for their unwavering commitment and hard work.

Last year, the Communications Working Group of Nuclear Medicine Europe once again dedicated itself to education and training in the field of nuclear medicine and molecular imaging. To this end, various measures were taken in 2024 to inform society, legislators and experts about the potential and challenges of the field.
On the one hand, the central website for the introduction to nuclear medicine has been updated to reflect current developments and innovations in the field. With the revised content, the site now provides a comprehensive first impression of its diagnostic and therapeutic possibilities.
However, the introduction of the interactive Hygieia in Q3 2024 was significantly more important in providing an overview of the field. For the first time, all radiopharmaceuticals currently approved in the European Union are presented at a glance. Users now have the option of not only sorting by desired subject areas or selecting different language versions, but also exporting their current display as a PDF. The consistently positive and extensive international feedback following the release of this useful tool encourages us to continue expanding it in the future.
In addition to focusing on what has already been achieved, it is of course important to also consider future prospects and challenges. With the steady growth of the field, a shortage of specialists is expected, which is already becoming apparent. Serge Goldman, nuclear medicine specialist, researcher and professor emeritus at the Université libre de Bruxelles (ULB), addressed this topic in two articles. He shared his views on Science, Significance, and Future Perspectives Of Nuclear Medicine as well as the question Why do medical students decide not to get trained in the most appealing specialty, nuclear medicine? Both articles form the basis for convincing aspiring young physicians and experts to choose the discipline. At the same time, they also serve to show society and legislators what obstacles exist on the path to becoming a nuclear medicine expert.
Furthermore, a new Group Co-Chair was elected and a centralized hub for the provision of digital resources for Nuclear Medicine Europe members was created.
The year ended with the in-person meeting of the working group in Brussels. Possible projects for 2025 and beyond were discussed on-site. The exchange with the Chairman of the Transport Experts Working Group of Nuclear Medicine Europe and the brief insight into the work of the Communications Working Group of top-10 European Association also stimulated exciting discussions.
“We would like to thank the members of the Communications Working Group for their support and contributions over the past year. Special thanks go to David Crunelle for the implementation of the numerous projects.”
Pascal Daniel and Jan Schöpflin
(Co-Chairs of the Communications Working Group)


The regulatory landscape in the EU is undergoing significant change with the proposed update to the pharmaceutical legislation by the European Commission (EC) in addition to the various guidelines and concept papers issued by the European Medicines Agency (EMA) during 2024. This year has been extremely active for the Regulatory Affairs and Quality Working Group and it is a testament to the WG members that we have been able to advise, propose, review and comment on a variety of topics to both EC and EMA on behalf of Nuclear Medicine Europe.
Nuclear Medicine Europe is the only EU industry body dedicated to the interests of companies working in radiopharmaceuticals and devices. This year, EMA formally recognised Nuclear Medicine Europe as a specialist stakeholder following our interactions with EMA over several years and our continuing commitment to collaboration on issues key to our industry. This recognition has paved the way for further engagement at various EMA-Industry Stakeholder meetings – thereby widening the reach of Nuclear Medicine Europe in helping Nuclear Medicine companies navigate EMA processes and procedures.
Radiopharmaceuticals are a hot topic in our industry and also for EMA who are drafting various guidelines and concept papers that are pertinent to drug development in this space. Guidance and legislation that have been in place since the early part of the century are under review and it is an incredibly exciting time for Nuclear Medicine Europe to be able to offer expert insights into these reviews to ensure that the development of radiopharmaceuticals reaches patients in need of these novel products.
During 2024, the Working Group continued to highlight challenges for the radiopharmaceutical industry with regard to the proposed EU Legislation update and submitted comments to EMA on the Development and Manufacture of Synthetic Peptides guideline. Various EMA concept papers and guidances regarding radiopharmaceuticals are anticipated in the first quarter of 2025 and will be addressed by the Working Group. Attendance at the new Industry Stakeholder meetings with EMA began in the last quarter of 2024 and the learnings discussed and disseminated within the Working Group. With over 30 members the Working Group is a vibrant community, working together to share the increasing workload of reviews and industry stakeholder meetings with EMA.


The Security of Supply Working Group once again played a vital role in coordinating international efforts to ensure a reliable supply of medical radioisotopes. The group – in particular the Emergency Response Team – mobilized quickly in both the spring and fall of 2024 to respond to delayed restarts/unplanned outages of the HFR reactor in the Netherlands in an effort to mitigate the loss of target irradiation capacity in these two periods.
The ERT provided prompt communications to international stakeholders regarding the nature and projected length of the supply shortages and worked with authorities to expedite the return to service of the affected facilities as soon as possible. The supply shortages were much more serious in the October-November period – reaching greater than 50% of normal demand – due to the fact that for a time no relevant research reactors were operating in Europe. However the situation was resolved with an earlier than planned return to service of both the HFR and Maria (Poland) reactors.
The Security of Supply WG also successfully coordinated the finalization of the reactor schedule for 2025 and provided important advice to both European and global studies on future demand for medical radioisotopes.
As evidence of Nuclear Medicine Europe’s role as the industry’s voice, the association was consulted on the recent 99Mo shortage. This is highlighted in publications such as “Why an Offline Nuclear Reactor Led to Thousands of Hospital Appointments Being Canceled” (Wired) and “Impact of radiopharmaceutical shortage prompts questions on UK resilience” (The Lancet Oncology).


This group is still young in the Nuclear Medicine Europe world.
For 2024 we enjoyed the arrival of representatives from several new members. As of today, we have 25 members sometimes willing to name a second deputy representative. This is to support and facilitate the involvement of the members. The association and Co-Chairs agreed to have member representatives name a deputy to attend meetings.
We also welcome Pr. Serge GOLDMAN, a long-time contributor to the activities of Nuclear Medicine Europe accepted to play the role of scientific referee and advisor for the activities of the Working Groupe.
Early 2024, the 2 Co-Chairs supported by Guy Turquet started 2024 with a brainstorming meeting in February aiming at defining the critical parameters, challenges and obstacles impeding the smooth and dynamic development of RLT and Theranostics. The gap between the number of eligible patients compared to those effectively treated remains a source of major concern for our members.
Different causes and effects were identified. Most of these conclusions have been confirmed throughout the year either during scientific meetings or reports.
This was presented to the group, generating feedback for the selection of strategic topics for future workshops. It is fair to say that we expected more responses, however, the following items were considered for primary review: healthcare cost/benefit ratio, infrastructure investments, safety regulations, and regulatory pathway.
These topics resonate particularly well with the renewed mission statement of the Working Group proposed in 2024; whose fundamental pillars are:
- Support the nuclear medicine industry in delivering innovative therapeutic solutions to healthcare professionals and patients.
- Establish radioligand therapy (RLT) as a key component among anti-cancer therapies.
- Promote equal patient access to the medical benefits of RLT across Europe.
- Make RLT affordable through its cost-efficient use of medical resources
In parallel, with the support of Pr. Serge GOLDMAN, we set the project of organizing a seminar with EU Health Attachés. Unfortunately, the calendar of the activities of the European Commission did not allow us to maintain the initial date of November 21st. The good point is the several positive messages from the Health Attachés to maintain the project but push it in 2025.
After a thorough review of the calendar of activities, it appears that June is the earliest period to consider. A certain number of members will be involved in this workshop. The program is set, a confirmation of the date is pending.
In late November Jean BONNET participated in the kick-off meeting of the JANE-2 project “WP10 – Hi-tech medical resources”, in Copenhagen. That will be another source of interaction and discussion among the team. At this stage we do not have the calendar for the next meetings in 2025. Adrien RAYMOND, will work with Jean on this project.
All actions and initiatives aim at eliminating the obstacles that Nuc Med specialists are facing when considering the choice of RLT for their patients. This justifies the continuity of actions for 2025.
- Sharing relevant data, reports or publication supporting expanded access to RLT in Europe info to maintain the best knowledge among the members and support effective contribution from the WG
- Nuclear Medicine Europe to issue a position statement for interactions with stakeholders at European and National levels
- Prepare the Health Attachés Meetings

The Transport Experts Working Group has made significant strides this year through strengthened collaboration, key publications, and productive meetings.
The increased cooperation with the Council on Radionuclides and Radiopharmaceuticals (CORAR) contributed to the revision of the IAEA Safety Standard SSR-6, aligning industry practices with updated regulatory requirements.
Two influential position papers were published:
• Recommendations on the IAEA SSR-6 revision.
• Addressing the lead ban in transporting radioactive materials, emphasizing safety and sustainability.
Two in-person meetings facilitated knowledge sharing and welcomed new members, enriching expertise and fostering collaboration. The Working Group remains committed to advancing safe and efficient transport practices, with gratitude extended to all contributors.
Nuclear Medicine Europe is very happy to have been able to contribute, through the Transport Experts Working Group, to achieving these positive outcomes. We extend our gratitude for the dedication and efforts of the group that made this possible.
The whole nuclear medicine community is deeply appreciative of the time and knowledge these teams contribute. Their dedication not only strengthens our organization but also helps drive impactful initiatives for the betterment of patient care. We look forward to another year of collaboration, innovation, and progress.
Newcomers in the Membership
Stronger together
We welcome six new members whose expertise spans innovative radioligand therapies, cutting-edge manufacturing, advanced logistics, and pioneering reactor operations. These organizations greatly contribute to the nuclear medicine and radiopharmaceuticals sectors, strengthening our collective impact on advancing healthcare and technology.
Explore the full list of Nuclear Medicine Europe Members by clicking here.
ARTBIO
Focused on developing innovative alpha radioligand therapies (ARTs) to treat cancer, the clinical-stage biotechnology company uses its proprietary AlphaDirect™ technology to deliver targeted therapies, utilizing the isotope Lead-212 (Pb-212) for precise tumor targeting while minimizing damage to healthy tissue. ARTBIO’s lead program, AB001, is currently undergoing human clinical trials. ARTBIO scientific legacy is rooted in nearly a century of pioneering work at the University of Oslo and Norway’s Radium Hospital.
LemerPax
Founded in 1970, the company offers innovative products including shielded containers, hot cells, and glove boxes tailored for safety and efficiency. Lemer Pax emphasizes customized solutions, leveraging advanced materials like tungsten and lead-free alternatives to ensure compliance with international safety standards. Its commitment to environmental responsibility and cutting-edge design underpins its mission to protect both users and the environment from radiation risks.
MURR – The University of Missouri Research Reactor
Operating as the most powerful university research reactor in the U.S., MURR runs 24/7 to produce radioisotopes used in cancer treatment, diagnostic medicine, and other therapeutic applications. It supports over 1.6 million patients annually, contributing significantly to advancements in nuclear medicine and radiopharmaceuticals.
PANTERA
PanTera Life, a collaboration between IBA and SCK CEN, focuses on producing actinium-225. By aiming for large-scale production of this isotope, PanTera seeks to advance targeted radiotherapy (TRT) and improve accessibility to innovative cancer treatments. Their approach combines advanced technologies and strategic partnerships to address the growing demand for actinium-225 in theranostics and clinical trials, with plans to begin production in the coming years.
PHSE
PHSE specializes in temperature-controlled logistics for the healthcare industry, ensuring safe and efficient transport of pharmaceuticals, biotechnology products, and advanced therapies. With over 20 years of experience and 29 global branches, PHSE delivers solutions like clinical trial logistics, radiopharmaceutical handling, and last-mile distribution, supported by innovative tracking technologies and strict regulatory compliance.
PMB Alcen
PMB, part of the ALCEN Group, is a specialized on the design and manufacturing of complex particle accelerator systems for various sectors, including medical, research, nuclear, defense, and industry. PMB develops systems such as automated radiopharmaceutical production for PET imaging, as well as advanced linear accelerators for radiotherapy and non-destructive testing.
Dr. Lutz Helmke's retirement
A new chapter begins

Dr. Lutz Helmke has made remarkable contributions to the medical technology and radiopharmaceutical industries throughout his career. Upon his departure from Nuclear Medicine Europe, he chose early retirement, marking the close of an impactful professional chapter and the start of a new phase in life.
Key Achievements
As a Board Member for the Radiopharma Segment at Eckert & Ziegler AG since 2018, Dr. Helmke has spearheaded significant advancements in radiopharmaceutical applications.
Industry Impact
Dr. Helmke’s efforts have been pivotal in addressing the global shortage of Actinium-225, a groundbreaking isotope for targeted cancer therapy. His leadership in this domain has positioned the industry closer to transformative innovations in oncology.
Career Highlights
Spanning leadership roles at organizations such as Abbott, St. Jude Medical, and MagForce AG, Dr. Helmke’s career exemplifies dedication to advancing medical technologies.
The entire Membership of Nuclear Medicine Europe thanks Dr. Helmke for his contributions and support in the association’s Executive Committee. We wish him every success and fulfilment in this new chapter of his life.
Heading to 2025
What's next?
With 2025 lying ahead of us, we are preparing for an exciting and challenging year. The geopolitical situation will reserve some surprises in different parts of Europe and the rest of the world. We will have the opportunity to connect to the activities of the newly elected European Commission and European Parliament. In 2024 announced company acquisitions and mergers will become a reality. Many big infrastructure projects in the nuclear medicine sector in Europe will make substantial progress. Some radiopharmaceuticals are waiting to be approved by the regulatory bodies.
A year of important news just started. Nuclear Medicine Europe is entering an election year, the time to elect the Executive Committee for a new triennium at the General Assembly.
The Working Groups have expressed the wish to meet more frequently in person. After two long COVID years, personal exchange has become a privilege and a special joy for our teams. The most creative way of working is, at the same table in a relaxed setting.
Working Groups are committed to increasing face-to-face meetings, incorporating sporadic insights from external consultants on specialized topics, and fostering stronger partnerships between groups on shared initiatives.
As the voice of the Nuclear Medicine industry, we are excited to advance the projects outlined and deepen our collaboration with all industry partners and policymakers in the year ahead
We are looking forward to a fabulous year together with all of you!
Mart-Jan, Konrade, Erich

Thank you!
Editor : David Crunelle