Nuclear Medicine Europe
Activity Report 2022
A word from the NMEU President
Mart-Jan Blauwhoff
Dear colleagues and friends of Nuclear Medicine Europe,
Another year over and to use the words of John Lennon: “…and what have you done?”.
These are turbulent times with the remnants of a pandemic, a war in Eastern Europe all causing besides actual pain also supply chain issues that influence our daily life and work environment.
But at the same time, Nuclear Medicine is making great strides towards a bright future with new theranostics coming up, an increasing recognition with referring physicians and regulators like EMA and European Institutions asking us for advice and collaboration.
On the latter, you will shortly get more information on our plans to have another symposium at the European Parliament as well as an exhibition on nuclear medicine in the Parliament’s building and increased collaboration with Euratom.
Nuclear Medicine Europe elected a new Executive Committee in June and as I stated at our networking party in Barcelona we intend to work as a team together with the administrative and creative team members and our Director General for the benefit of our association and you, our valued members.
One of the mainstays of our activities is our working groups who, despite ongoing travel difficulties, again this year had some success stories. Our Regulatory WG had a very fruitful meeting with EMA and another one is planned for early 2023. Therapy WG is working with external support to bring the message about therapeutic Nuclear Medicine across, Security of supply and ERT had a lot on their hands trying to solve supply issues, just to name a few highlights. I would on behalf of the ExCo, therefore, like to thank all WG members for their work and continued support as well as the companies who allow these persons to spend time and effort on Nuclear Medicine Europe activities.
In the meantime, we are delighted to present our annual newsletter – and from the ExCo and the “Brussels” team, we wish you all a happy holiday season and a wonderful 2023!
NMEU Position Paper
Our message to the EU Institutions
Publication / EU Affairs
Over the past three years, the European Union has grappled with the Covid-19 pandemic and the war in Ukraine, while rolling out its ambitious programmes for green and digital transitions. Amid all this, it is important not to lose sight of our health challenges, which is why Nuclear Medicine Europe moved to raise its profile with the EU institutions through a position paper mapping out why nuclear medicine matters, and how Europe can support it. It reflects the combined views of the association’s 45 members on how support from both EU and local politicians will be vital for ensuring a stable and secure treatment of patients in the member states.
The seven-page paper offers a concise and user-friendly summary of nuclear medicine for policymakers, explaining how it has been a vital imaging and therapeutic technique for almost a century, and how it is more precise and sophisticated than ever today, thanks to a range of modern technologies from fields such as medical physics, radiochemistry and informatics.
“Sophisticated medical devices, called PET and SPECT, detect these particles to create functional images of internal processes. They can capture physiological or pathological pathways directly related to the disease status of the patient,” the paper says. “Nuclear medicine has a huge potential to diagnose and treat patients. However, many issues must be addressed if it is to fulfil its promise in Europe and elsewhere.”
The position paper, adopted at the Nuclear Medicine Europe AGM in June 2022, and published shortly afterwards, pushed for action at EU level in four areas: secure and reliable supply for current and future radioisotopes; regulation that adapts to the specificities of radiopharmaceuticals; support for innovation and technical development across the EU; and awareness-raising about nuclear medicine’s benefits.
Specifically, Nuclear Medicine Europe calls for periodical reviews of payment rates and payment policies, ensuring the European Medicines Agency (EMA) has an in-house nuclear medicine expert and supporting international and cross-sectoral education in nuclear medicine. Our primary goal, however, is to bring together experts to produce informed advice about how nuclear medicine can improve patient care within Europe – including the main EU policymakers.
As explained in details in the position paper, and in order to briefly explain to the legislative bodies the main challenges the nuclear medicine industry is facing, support slides were created and presented during the last NMEU Symposium.
A busy year for the Security of Supply Working Group
War and other disruptions kept the SoS Working Group busy
Working Group / Association / Networking
The Nuclear Medicine Europe Security of Supply Working Group (SoS WG), and Emergency Response Team (ERT), play a key role in helping to promote European and global security of supply of medical radioisotopes.
The Security of Supply WG oversees a yearly effort to coordinate isotope-producing reactor schedules, navigating between both monthly refueling needs and periodic extended, preventive maintenance outages required of all such reactors

In 2022, the SoS WG took part in several key events. In June, it was at the Society of Nuclear Medicine and Molecular Imaging (SNMMI) annual general meeting in Vancouver, its first in-person meeting since 2019. There was also a virtual meeting in September to discuss potential adjustments to the 2023 Rev. 2 schedule. In addition, the Security of Supply WG sent letters sent to several reactors requesting schedule changes from their initial plans, and the reactor schedule 2023 was approved at the EANM Barcelona in October 2022.
The year was dogged by isotope supply issues and disruptions, which prompted the Security of Supply WG to issue communications on the IRE HEU line outage, on I-131 supply, and the MARIA research reactor maintenance. There were also several meetings with the Emergency Response Team (ERT), notably in regard to an unplanned outage of the HFR reactor in January-February 2022 and an unplanned outage of the BR2 reactor that occurred in October – November 2022. After the war in Ukraine erupted, the Security of Supply Working Group provided information and advice in regard to Russia’s isotope supply and contributed to international discussions on potential sanctions. Finally, the Security of Supply WG contributed to and participated in EU SAMIRA studies, providing advice and assistance for the SAMIRA Action Plan and the European Radioisotope Valley Initiative on emerging isotope requirements.
Emergency Response Team called into action
A monitoring expert group
Monitoring / Reactors
The Emergency Response Team (ERT) mobilises key stakeholders to respond to unanticipated outages of reactors and isotope processing facilities, encouraging mitigating measures where possible, and providing timely communications to the international community on supply chain disruptions and risks to adequate international isotope supply.
The Security of Supply WG also provides expertise and advice to the European Commission regarding both short and long-term isotope supply matters, including risks arising from the illegal Russian aggression against Ukraine on the supply of Russian isotopes, and new isotope requirements – and related-isotope producing infrastructure – from new developments in nuclear medicine and healthcare.


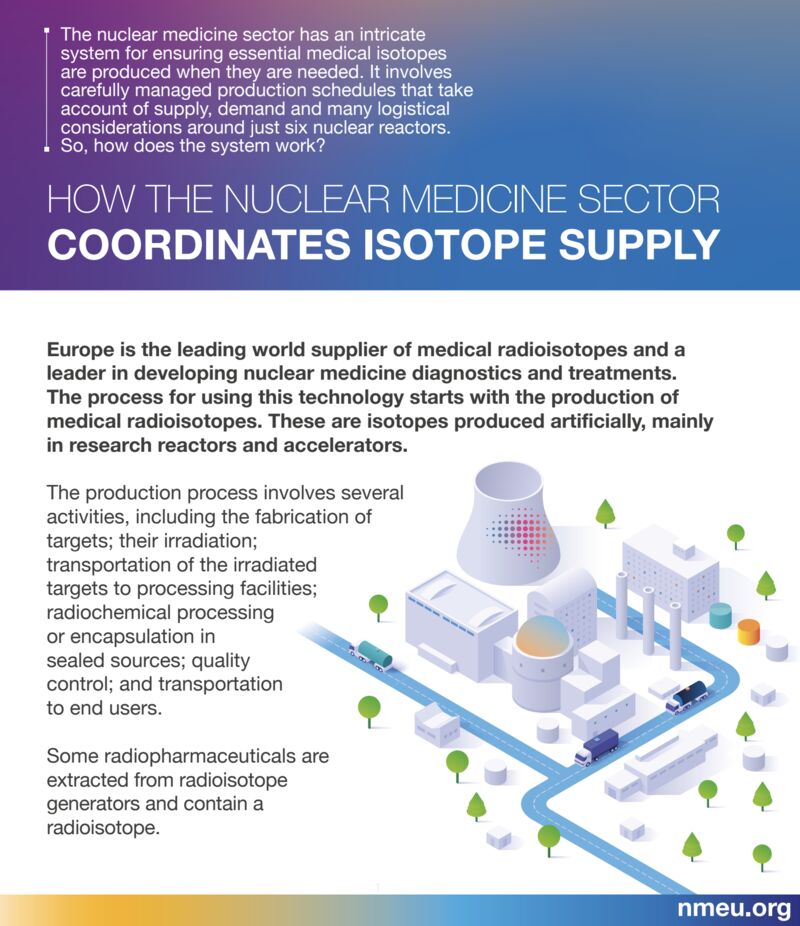
How the nuclear medicine sector coordinates isotope supply
New paper published
Publication / Communication
In the face of a supply chain crunch for vital products and specialised equipment Nuclear Medicine Europe published a position paper mapping out the challenges and the possible solutions. The paper looks at the process for using nuclear medicine technology, from the production of medical radioisotopes to their delivery to radiopharmaceutical product manufacturers, and their shipping to medical institutions.
“The nuclear medicine sector has an intricate system for ensuring essential medical isotopes are produced when they are needed,” the paper says. “It involves carefully managed production schedules that take account of supply, demand and many logistical considerations around just six nuclear reactors.”
The production process entails several activities, including the initial production of the targets; an irradiation process; transporting the irradiated targets to processing facilities; radiochemical processing or encapsulation in sealed sources; quality control; and shipping to end users.
Europe is the leading world supplier of medical radioisotopes and the entire supply chain process needs careful planning. The coordination, collaboration and monitoring are overseen by a neutral platform, the Security of Supply Working Group, which ensures all the key actors align their agendas.
European Observatory
on the supply of medical radioisotopes
ESA + NMEU

The European Observatory on the Supply of Medical Radioisotopes, which assesses, monitors and supports the EU supply of medical radioisotopes with an emphasis on the most vital Molybdenum-99/Technetium-99m (Mo-99/Tc-99m), had a busy 2022.
Chaired jointly by Nuclear Medicine Europe and the Euratom Supply Agency (ESA), the Observatory brings together various supply chain stakeholders (industry, research organisations and EU member states), nuclear medicine organisations, Nuclear Medicine Europe members, international organisations, as well as relevant European Commission services and agencies.
After the Observatory’s mission statement was revisited in 2021 and new terms of reference drafted, it opened the way for increased collaboration by all the actors involved in the supply chain. The group membership was extended to EU member states, giving national governments access to the expertise and information they need for their strategies and policies.

The Observatory’s plenary meeting on June 29, 2022 in Brussels also marked its tenth anniversary and gathered around 50 participants.
The meeting was largely devoted to the impact on the supply of medical radioisotopes due to the Russian military aggression in Ukraine. A panel discussion on the security of supply gathered representatives from NMEU, ESA, the European Medicines Agency (EMA) and the European Association of Nuclear Medicine (EANM).
It was followed by a debate on the possible production of stable isotopes in the EU, with URENCO and ORANO presenting their projects to start domestic production of target materials, namely, Mo-98, Mo-100 and Yb-176, isotopes that are currently supplied almost exclusively by Russia.
The session ended with the presentation by the ESA Advisory Committee Working Group on European production of High-Assay Low-Enriched Uranium (HALEU) of their recent HALEU report. HALEU supply is currently dependent on both Russia and the US, but the Russian aggression in Ukraine could threaten supply of this critical material. If this occurs, it will put patients in danger and put the EU’s dominant position in medical radioisotope production at risk. The report identified options to provide different levels of the security of HALEU supply in the EU.
The keynote speech at the Observatory plenary was delivered the MEP Bartosz Arłukowicz, the chair of the European Parliament’s Special Committee on Beating Cancer (BECA), who detailed the results of the committee. The next Observatory plenary is set to take place in 2023.
The Observatory – with crucial NMEU input – helped the ESA and the relevant EU bodies to mobilise in time to mitigate potential risks to supply chains in 2022. This was the case when it seemed likely there could be a shortage of I-131: ESA promptly informed both the EMA and the EU Health Security Committee (HSC), which is mandated coordinate and share information on national preparedness activities.
The EMA then alerted the Coordination group for Mutual Recognition and Decentralised Procedures – Human (CMDh) about potentially changing the terms of marketing authorisation of I-131 from HALEU targets. A work-sharing procedure was agreed to ensure a coordinated approach and head off duplicate evaluations by individual competent authorities. In parallel, the EMA asked the national single point of contact (SPOC) network to conduct the criticality assessment at the national level to get a detailed view of the impact of the potential shortage. These combined actions ensured that they reduced the risk of a I-131 shortage, which could have had a devasting impact on the supply of this vital isotope.
2019-2022 newsletter
The improbable triennum
Publication

As Antonis Kalemis’s stepped down as Nuclear Medicine Europe President ended in June 2022, we paid tribute to his three-year term with a special edition of our newsletter. Antonis took on the presidency in mid-2019 and he steered Nuclear Medicine Europe through an extraordinary period. “When the Covid-19 pandemic plunged the whole world into the unknown in early 2020, we all had to be creative,” he wrote. “We had to develop new tools and new ideas, to defend the nuclear medicine industry. We had to respond to the urgent issues facing our members and try to solve them. It was not easy, but necessity is the mother of invention.”
By the end of his three-year term, Nuclear Medicine Europe was engaging fruitfully with Europe’s decision-makers, the medical world and the general public – and also opened its doors to 10 new members and associate partners.
NMEU industry news
A more effective newsletter format
Publication / Communication
In an era where so much of our time is spent staring at screens and tapping on apps, we needed to ask whether the traditional newsletter is a dying format. Not yet, it seems. But it can be improved.
In our case, the information about the nuclear medicine industry could be refocused on its effectiveness and presented in a more user-friendly manner – including direct hyperlinks – and sent every other week. It should be read in less than one minute, detailing only what is relevant. Links to Selected Industry News allow members to quickly dive into essential market news and clinical news from the previous two weeks that they may have missed.
After a successful pilot project, we turned the newsletter into a fortnightly must-read for Nuclear Medicine Europe members.
In collaboration with the Communications Working Group.

Building our Linkedin presence
How we made our mark in social media
Communication
It is difficult for any organisation – a business, an agency, an association, an NGO – to ensure its visibility without having a presence on social media. Nuclear Medicine Europe was entirely absent in this field until March 2020. However, through a carefully crafted strategy, we developed our LinkedIn account, creating a communication platform to reach our members, the academic world and the wider nuclear medicine industry.
We knew that growing our presence and engagement on social media would require more than just posting links and comments, and hoping it will grow by magic. The reality is more complicated, especially in a system where paid promotional posts are often the key drivers for traffic.
Nonetheless, we worked to grow our channel organically. Today, the carefully managed Nuclear Medicine Europe Linkedin account is our dedicated channel for our communication about our activities. While NMEU’s resources for community management are scarce, we have worked to ensure a consistent approach in our strategy, curated by our senior communication advisor, who has long experience running social media campaigns for global brands.
Two years after setting up our LinkedIn account, we have over 2,000 followers – with an increase of more than 1,200 in 2022 alone.
Below is an overview of our audience on this platform that has become an essential communications tool in today’s world.
Linkedin followers
Quick data
In 1 year (11.2021 – 11.2022) :
• +1285 new followers
• 1,348% increase in Reactions
• 487% increase in Reposts
• 100% organic growth, 0% sponsored
• Average of 2000 impressions per post
• Average of 120 clicks on links
Job functions
Industry

Refreshing a 12-year-old website
Giving a new look to nuclear medicine’s general public site, whatisnuclearmedicine.com
Communication / Awareness
Twelve years ago, we set up our website to explain the basics of nuclear medicine to people outside the sector: whatisnuclearmedicine.com It was a great introduction to the issue, and it proved very successful with its pedagogical animations, a useful educational tool for the general patient. Indeed, it is often used as the entry point for nuclear medicine to a broad audience. But it was high time to revamp the site to fit modern standards. The new version is now ready.
Annual General Meeting
A networking dinner
Events / Networking
As the Covid-19 pandemic retreated across much of Europe, many of government restrictions were lifted. That meant Nuclear Medicine Europe could hold physical meetings again, including our Annual General Meeting (AGM), which took place in the prestigious and historic Château St-Anne in the Brussels suburb of Auderghem.
The AGM was a much-needed reconnection for our members, and the dinner offered a valuable networking opportunity. Here are some pictures from the event.
2022-2025 Executive Committee begins work
New team elected
Communication / Association

The Nuclear Medicine Europe 2022-2025 Executive Committee was elected at the end of the 28 June Annual General Assembly in Brussels.
Congratulations to (from left to right) the new Nuclear Medicine Europe President Mart-Jan Blauwhoff (Curium Pharma), Vice-President Dr. Lutz Helmke (Eckert & Ziegler AG), Vice-President & Treasurer Dr. Konrade von Bremen (SWAN Isotopen AG), Vice-President and Secretary General Erich Kollegger (IRE – Institute for radioelements).
Members talk about NMEU
Introductory video explains our work
Communication / Association
In a four-minute video, our members gave testimonials explaining the benefits – professionally and personally – of Nuclear Medicine Europe and its working groups. The film featured Mustafa Aydın Küçük from Monrol Europe, Matthew Morrison from Blue Earth Diagnostics, Nuclear Medicine Europe President Mart-Jan Blauwhoff from Curium Pharma, Revital Melzer fromGE Healthcare, Jayne Senior fromANTSO, Christiana Gameiro from IBA RadioPharma, Emiliano Spagnolo fromComecer Group, Lutz Helmke from Eckert & Ziegler, and Julia Moebius from Rotem GmbH.
We want to thank all of them for being so ready to take part in our film, and for explaining so concisely the value of Nuclear Medicine Europe.
NMEU at SNMMI
Presence at North American event
Congresses / Events / Networking
After a pandemic-induced hiatus, the North American-based professional association, the Society of Nuclear Medicine and Molecular Imaging (SNMMI), held its annual congress in Vancouver. As in previous years, Nuclear Medicine Europe took part too: we had a space dedicated to members for meetings and networking.
A Security of Supply meeting was held in the margins of the event, Nuclear Medicine Europe took part in the annual Council on Radionuclides and Radiopharmaceuticals (CORAR) meeting which takes place every year during the congress, where both associations can share crucial information about their activities and their plans.





EANM Congress in Barcelona
Presence at the European congress
Congresses / Events / Networking
After a three-year break, the European Association of Nuclear Medicine (EANM) held its annual congress in October in Barcelona.
This edition was very successful, with a big attendance. Nuclear Medicine Europe had a huge space in the center of the main hall of the congress entirely dedicated to members for meetings and networking,
The EANM was also the scene for a ‘Get Together’ meeting, an exclusive networking event held at the Puro Beach section of the Hilton Diagonal Mar. Some 80 people, members and partners gathered to meet and bounce ideas, reconnect with past collaborators and have informal discussions outside of their booths within the congress.





Heading to 2023
What's next?

The Nuclear Medicine team and Working Group members have already started work on next year’s projects and activities.
In addition to the Working Group meetings, publications, and participations, we will also take part, as always, at the EANM Congress in beautiful Vienna. We are also planning a very special event in the European Parliament, as well as the next edition of the Nuclear Medicine Europe Symposium.
We look forward to telling you more very soon!


Thank you!
This annual activity report has been made in collaboration with Ira Goldman, Remigiusz Baranczyk for the European Observatory, Mart-Jan Blauwhoff and Leo Cendrowicz.
Editor : David Crunelle